METHYLENE CHLORIDE
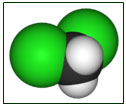
Chemical Formula : CH2C12 CAS Registry Number 75-09-2
Dichloromethane (DCM or methylene chloride) is the organic compound with the formula CH. This colorless, volatile liquid with a moderately sweet aroma is widely used as a solvent. More than 500,000 tons were produced in 1991. Although it is not miscible with water, it is miscible with many organic solvents. It was first prepared in 1840 by the French chemist Henri Victor Regnault, who isolated it from a mixture of chloromethane and chlorine that had been exposed to sunlight.
Uses
Dichloromethane's volatility and ability to dissolve a wide range of organic compounds makes it a useful solvent for many chemical processes. Concerns about its health effects have led to a search for alternatives in many of these applications. It is widely used as a paint stripper and a degreaser. In the food industry, it has been used to decaffeinate coffee and tea as to prepare extracts of hops and other flavorings. Its volatility has led to its use as an aerosol spray propellant and as a blowing agent for polyurethane foams.
Specialized uses
Dichloromethane chemically welds certain plastics, for example, it is used to seal the casing of electric meters. Often sold a main component of plastic welding adhesives it is also used extensively in the model-making industry for joining plastic components together — it is commonly referred to as "Di-clo."Also used in the garment printing industry for removal of heat-sealed garment transfers. Usually sold in cans with methanol as the propellant gas. Its volatility is exploited in novelty items — drinking birds, bubble lights, and jukebox displays.Dichloromethane is used within the material testing field of civil engineering, specifically it is used during the testing of bituminous materials as a solvent to separate the binder from the aggregate of an asphalt or macadam to allow the testing of the materials.