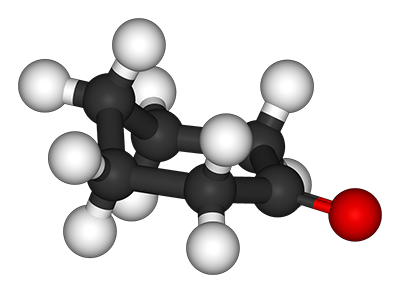
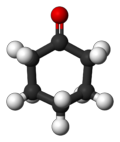
CYCLOHEXANONE (C6H10O)
 |
 |
 |
Description :
Production: Cyclohexanone is produced by the oxidation of cyclohexane in air, typically using cobalt catalysts:[4]C6H12 + O2 → (CH2)5CO + H2OThis process co-forms cyclohexanol, and this mixture, called "KA oil" for ketone-alcohol oil, is the main feedstock for the production of adipic acid. The oxidation involves radicals and the intermediacy of the hydroperoxide C6H11O2H. In some cases, purified cyclohexanol, obtained by hydration of cyclohexene, is the precursor. Alternatively, cyclohexanone can be produced by the partial hydrogenation of phenol:C6H5OH + 2 H2 → (CH2)5COThis process can also be adjusted to favor the formation of cyclohexanol.
Uses : The great majority of cyclohexanone is consumed in the production of precursors to Nylon 6,6 and Nylon 6. About half of the world's supply is converted to adipic acid, one of two precursors for nylon 6,6. For this application, the KA oil (see above) is oxidized with nitric acid. The other half of the cyclohexanone supply is converted to the oxime. In the presence of sulfuric acid catalyst, the oxime rearranges to caprolactam, a precursor to nylon 6: |

Chemical Shape
|
|||
|
HAZARDS
NFPA 704 EU Classification |
|||


