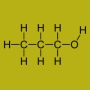
Propan-1-ol is a primary alcohol with the molecular formula of C3H8O. It is also known as 1-propanol, 1-propyl alcohol, n-propyl alcohol,n-propanol, or simply propanol. It is an isomer of propan-2-ol. It is used as a solvent in the pharmaceutical industry, and for resins and cellulose esters. It is formed naturally in small amounts during many fermentation processes.
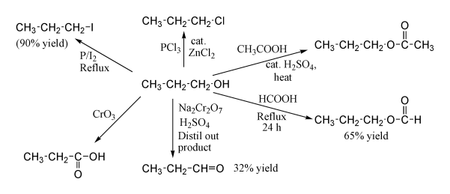
1-Propanol shows the normal reactions of a primary alcohol. Thus it can be converted to alkyl halides; for example red phosphorus and iodine produce n-propyl iodide in 90% yield, while PCl3 with catalytic ZnCl2 gives 1-chloropropane. Reaction with acetic acid in the presence of an H2SO4 catalyst under Fischer esterification conditions gives propyl acetate, while refluxing propanol overnight with formic acid alone can produce propyl formate in 65% yield. Oxidation of 1-propanol with Na2Cr2O7 and H2SO4 gives only a 36% yield of propionaldehyde, and therefore for this type of reaction higher yielding methods using PCC or the Swern oxidation are recommended. Oxidation with chromic acid yields propionic acid.



Hazards :
S-Phrase:(S2), S7, S16, S24, S26, S39