TOULENE
Chemical Formula : C6H5CH3 CAS Registry Number 108-88-3
Toluene, also known as methylbenzene, or Toluol, is a clear water-insoluble liquid with the typical smell of paint thinners. Chemically it is mono-substituted benzene derivative (benzene derivative in which one hydrogen atom from the benzene molecule has been replaced by a univalent group; in this case CH3).It is an aromatic hydrocarbon that is widely used as an industrial feedstock and as a solvent. Like other solvents, toluene is also used as an inhalant drug for its intoxicating properties; however, this causes severe neurological harm.
In the industry, hexanes are used in the formulation of glues for shoes, leather products, and roofing. They are also used to extract cooking oils from seeds, for cleansing and degreasing all sorts of items, and in textile manufacturing.A typical laboratory use of hexanes to extract oil and grease contaminants from water and soil for analysis. Since hexane cannot be deprotonated, it is used in the laboratory for reactions that involve very strong bases, such as the preparation of Grignard reagents.
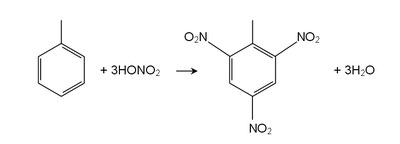
| Toluene reacts as a normal aromatic hydrocarbon towards electrophilic aromatic substitution. The methyl group makes it around 25 times more reactive than benzene in such reactions. It undergoes smooth sulfonation to give p-toluenesulfonic acid, and chlorination by Cl2 in the presence of FeCl3 to give ortho and para isomers of chlorotoluene. It undergoes nitration to give ortho and para nitrotoluene isomers, but if heated it can give dinitrotoluene and ultimately the explosive trinitrotoluene (TNT). |
 |
Application
|
 |
||