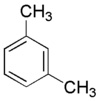
M-Xylene
Chemical Formula : C6H4(CH3)2 CAS Registry Number : 1330-20-70
Description
Xylene is a clear, colourless, aromatic hydrocarbon liquid of characteristic odour.
Xylenes are extracted or distilled from reformate, a stream derived from the refining of high-octane motor gasoline. They can also be produced from toluene using the disproportionation process. They are colourless, sweet-smelling liquids that are very flammable.Xylenes occur as three isomers, each having two methyl groups attached to a basic benzene hydrocarbon ring. The type of isomer distinguished by the position of the methyl groups on the ring. Para-xylene has the methyl groups attached on opposite sides of the ring, ortho-xylene has the two methyl groups next to each other, while meta-xylene has them positioned with one carbon in between the two groups. Shell chemicals companies primarily supply xylenes as a mixed stream, although various processes can be used to separate and/or convisomers.
Applications
Some mixed xylenes are used as solvents and in the printing, rubber, and leather industries. However, most mixed xylenes are separated and the individual isomers consumed in specific end-uses. Para-xylene is primarily used as a feedstock for terephthalic acid, a key component in polyethylene terephthalate (PET) resins. Ortho-xylene is used in plasticisers, medicines, and dyes.Mixed xylenes are also a desirable gasoline component, but are blended less often than toluene because there is greater demandvalue in their chemical applications.
|
Chemical Structure |
 |
NFPA 704 |
R-phrases: R10, R20, R21, R38
S-phrases: S25
|